Explain the Difference Between Density and Specific Gravity
Therefore these terms depends on. Bulk density is the ratio between soil weight to the total volume.
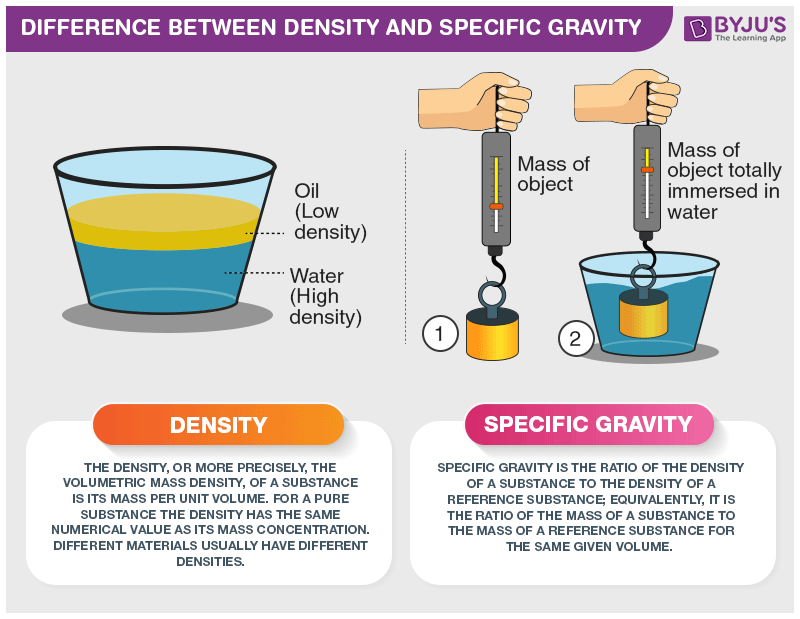
Difference Between Density And Specific Gravity With Its Practical Applications In Real Life
Specific Gravity The specific gravity SG of a substance is the ratio of the density ρ of the substance to the density of a reference substance at a specific condition ρ ref.
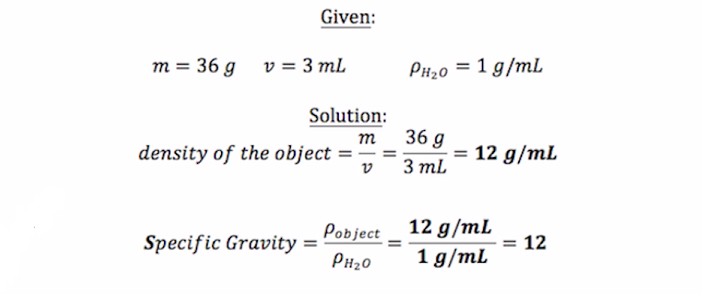
. We take the density and SG of water as 1. The most common reference for solids and liquids is water at 40C which has the following density. The difference between density and specific gravity is that one is a ratio of the other density is the measure of the amount of mass per volume.
On the other hand density is expressed in terms of kgm 3 SI. It is denoted by the symbol S. Specific gravity although the name suggests something related to gravity.
Density is defined as mass per unit volume. The density is defined as the mass of a substance per unit volume. When compared to density specific gravity does not have an SI unit.
Name three constituents that might be present if a urinary tract infection exists. Specific gravity S density of liquid density of water density of gas density of air The density of water is taken as 1000 kg cubic metre. The main difference between density and specific gravity is that density is the mass per unit volume of the substance whereas specific gravity is a ratio comparing the density of one substance to the density of another reference substance.
1density is the mass per unit volume while the specific gravity is the ratio of density of material to the density of some referenced material. True specific gravity is the ratio of the density of a sample by the density of water whereas apparent specific gravity is the ratio of the weights of. 6 rows Density is the specific property of matter and it uses the ratio of mass to the volume of.
Multiplying the specific gravity by t. The Twaddle scale is a simplified scale based on specific gravity where 0 Twaddle equates to SG6060F of 100 that of water and each degree Twaddle equals 0005 SG. There is a noticeable difference between density and specific gravity even though both are used to represent mass and are used to compare different substances.
The specific gravity 6060F scale compares the relative density of a given liquid with that of water at 60F 156C. It has the SI unit kg m -3 or kgm 3 and is an absolute quantity. For liquids water is taken as the standard fluid where as for gases air is taken as the standard fluid.
Specific gravity which is also called as relative density is a measure of density with respect to a density of pure water. See full answer below. Density of a material.
Being a ratio the relative density has no unit. While density is expressed as an absolute term specific gravity is considered a relative term. Specific gravity is defined as the mass of a unit volume of the given material divided by the mass of the unit volume of the reference material.
For gases the specific gravity is normally calculated with reference to air. I apparent specific gravity gross apparent specific gravity ii apparent density iv gross apparent density of fine. Explain the difference between apparent specific gravity and gross apparent specific gravity material for aggregates.
Difference between density and specific gravity. Explain the relationship between the color specific gravity and volume of urine. The density of a material tells you how close the molecules are packed and how heavy the molecules are.
Density and specific gravity are both indications of how much mass a substance would occupy in a given volume. Specific gravity is the ratio of a materials density with that of water at 4 C where it is most dense and is taken to have the value 999974 kg m -3. When measuring the density and specific gravity we use the masses of materials.
Specific gravity for gases is defined as the ratio of the density of the gas to the density of air at a specified temperature and pressure. Just like density temperature and pressure have an influence on specific gravity. 6 rows The main difference between density and specific gravity is its use in measuring this mass of a.
The density of air at room temperature is 120 kgm 3. And this is why specific gravity does not have a standard unit. What are the Similarities Between Density and Specific Gravity.
The density of a substance is defined as the ratio of its mass and volume. Density is the ratio of mass to volume of a material and specific gravity is the ratio of weight to volume of a material. Density is the mass per unit volume of a material while specific gravity is the density of a material divided by the density of a water.
Density is the property of matter represented by a ratio of mass to a unit volume of matter. When the reference substance is water in case of solids or liquids and hydrogen in case of gases the relative density is referred to as specific gravity. 1000 gcm 3 1000 kgm 3 6243 lb m f 3 The density of a liquid or solid in gcm 3 is numerically.
Generally the smaller the volume the greater the specific gravity more solutesvolume and the deeper the color. Specific gravity is a comparison of the density of an object with the density of water 100 gmL at 4 degrees celsius. The term specific gravity of soil refers solely to the solid matter while bulk density includes the pore volume of an aggregate.
2 density is absolute term but specific gravity is relative term as it is the value which is referenced with others.

Frequently Asked Questions What Is Specific Gravity

Density And Specific Gravity Youtube

Difference Between Density And Weight Compare The Difference Between Similar Terms

Comments
Post a Comment